NCATS Scientists Prioritize Compounds to Advance Research on Mitochondrial Damage
October 10, 2018
Mitochondria are tiny structures inside cells that produce the energy needed to carry out a cell’s daily biological tasks. But some environmental chemicals can damage mitochondria and lead to health issues such as heart disease, diabetes, cancer, and neurodegenerative disorders.
Through the Toxicology in the 21st Century (Tox21) program — a collaborative effort among NCATS, the National Toxicology Program, the Environmental Protection Agency, and the Food and Drug Administration — scientists work to improve laboratory testing methods to identify toxic chemicals and predict their toxicity in humans. A primary focus is to find new and more efficient ways to evaluate thousands of compounds that could potentially damage mitochondria.
Several years ago, Tox21 scientists combed through approximately 8,300 chemical compounds, finding 622 that potentially disrupted mitochondrial activity to varying degrees. The scientists used a format called high-throughput screening, which enabled them to test thousands of compounds at once, to examine the effects of the compounds on mitochondrial function.
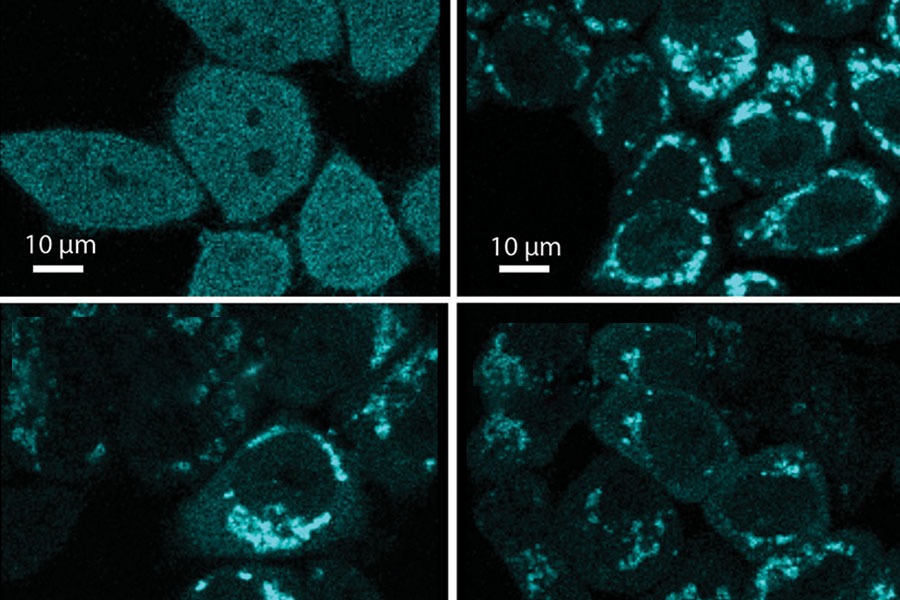
Three chemicals (FCCP, chlorfenapyr and pinacyanol) cause damage to the cell’s mitochondria. An enzyme then facilitates the recycling of the damaged mitochondria, as shown in the top right and bottom two panels. Normal cells are shown in the upper left panel. (Environmental Health Perspectives and Nuo Sun, Ph.D., Photo)
“Using animal models to evaluate thousands of compounds would be expensive, inefficient and take many years to complete,” said Menghang Xia, Ph.D., a lead in the Systems Toxicology lab for the Tox21 program at NCATS. “We would like to prioritize these compounds and choose a few compounds in the end to study in other models and eventually generate a computational model that allows us to screen them more efficiently.”
In a study published in Environmental Health Perspectives, Xia and her colleagues devised such an approach. They conducted tiers of assays, or tests, to identify and prioritize compounds according to their ability to disrupt mitochondrial activity. Using this strategy, scientists narrowed the list to 34 compounds and ultimately determined that four poorly characterized chemicals should be investigated in greater detail.
Most of the assays were already in use but were modified for use in NCATS’ high-throughput research facility, enabling many assays needed for this analysis to be conducted in weeks. In comparison, most animal studies take months to complete.
“This is the first time a combination of tests has been developed and used to flag chemicals that can potentially disrupt mitochondrial function, and, at the same time, also help us to understand how such disruptions occurred,” said co-author Anton Simeonov, Ph.D., scientific director of the NCATS Division of Preclinical Innovation.
The tests were wide-ranging. For example, the first key screening test measured the effects of compounds on mitochondrial “membrane potential,” which can indicate the impact on a cell’s ability to generate and process energy.
“The mitochondrial membrane potential is one of the best ‘canary in the coal mine’ tests for chemicals that could wreak havoc on mitochondria,” Simeonov said.
Study co-authors with the National Toxicology Program examined how compounds’ effects on mitochondrial function could affect the development of a commonly used laboratory model, the roundworm, while FDA collaborators showed how compounds might affect needed oxygen levels in mitochondria. Researchers also studied how compounds’ toxic effects on mitochondria affected human neuronal stem cell activity and the levels of a protein that is associated with DNA damage and many different cancers.
Xia noted that a similar approach can be used as a template for other large-scale toxicity studies. In addition, the use of human cells in initial tests could help make the approach more predictive in people.
The researchers hope that computational scientists will use these data to generate predictive computer models. In the meantime, Xia and her colleagues would like to test — and unravel — how such compounds might affect genes that control mitochondrial activity.