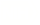Bold and Rigorous Approaches
NCATS addresses ambitious research questions with rigorous and robust methods that generate reproducible findings and advance translation.
3-D Model for Rare Neuromuscular Disorders Speeds Clinical Trial
For people with rare diseases, the road to effective treatments can seem endless. But a tiny device that recreates how human tissues and organs work could help those with two rare, devastating neuromuscular diseases find a shorter route to new therapies.
Chronic inflammatory demyelinating polyneuropathy and multifocal motor neuropathy cause the immune system to attack nerve cells and disrupt messages from the brain to the muscles. Current treatments can help but often are inconsistent.
Traditional drug development tools, such as two-dimensional cell cultures and animal models, don’t accurately predict how people will respond to an experimental therapy. As a result, about 90% of promising therapies fail when they reach clinical trials in people.
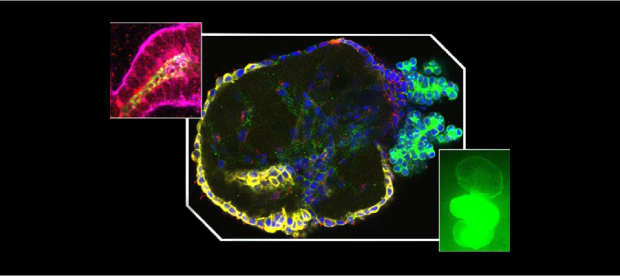
A scientific team supported by NCATS took a bold approach to boost the odds for a promising drug that targets these two neuromuscular diseases. The researchers created a tiny, bioengineered three-dimensional model that uses human tissue to better mimic how the two neuromuscular diseases take their toll in humans. Such organ-on-a-chip models, or “tissue chips,” allow researchers to rigorously test a new drug and more accurately predict how it will work when it reaches clinical trials in people.
To simulate the diseases, the research team created a tissue chip model with motoneurons, which transmit messages from the brain to muscles, and Schwann cells, which help the signals move more quickly. The scientists then exposed the cells to blood serum from people with the two rare diseases. That exposure caused a shower of immune system antibodies against the cells, slowing down the motoneuron signals.
The scientists treated their tissue chip model with a drug that blocks the faulty immune system reaction. With the drug, the cells and the message speed returned to normal.
This tissue chip study provided key preclinical data for a company developing the drug to receive U.S. Food and Drug Administration (FDA) authorization to test the drug in a clinical trial. This work marks one of the first examples of scientists using primarily tissue chip data for an FDA Investigational New Drug application to test the efficacy of a candidate drug in people with rare diseases.
“Tissue chip technologies have tremendous implications for drug discovery and the development of more effective medicines to treat rare and common diseases,” said Danilo Tagle, Ph.D., director of the Office of Special Initiatives at NCATS. “This system is already demonstrating its utility in modeling rare diseases and as an alternative to animal models in determining the effectiveness of therapeutics.”
Learn more about how NCATS uses bold and rigorous approaches with technology like tissue chips to study diseases and test therapies quickly to better predict which ones will move from the laboratory to successful clinical trials in people.
More Approaches Stories
Building Lung Tissue Models to Speed Virus Drug Testing
New 3-D Model Offers Insights into Role of Glucose in a Deadly Kidney Disease